Aquarium Lab Report
Title: My Aquarium
Purpose: to monitor a freshwater ecosystem and to collect/ interpret data
Materials:
- pitcher
- plug
- cup
- water
- tank (mine is a 1.5 gallon)
- dechlorinator
- bacteria
- gravel
- light
- decor
- live aquarium plants
- hiding spot
- ammonia test kit
- filter
- nitrite test kit
- food
- towels
- fish net
- food
- strainer/siv
- 4 guppies (male)
Procedure (steps):
- clean tank/ aquarium
- use strainer/ siv with warm water to clean gravel
- rinse aquarium/tank with water (NO SOAP)
- place gravel and decor in empty aquarium
- gravel first
- decor second
- use pitcher and fill aquarium with tap water
- pour gently so you don't agitate the gavel and decor
- if water spills, use paper towels
- add dechlorinator and bacteria
- wait 24 hours so aquarium can stabilize
- test water with nitrite and ammonia test kit
- if tests register "OK" add fish
- pour guppies into fish net
- immediately add fish to aquarium ( 4 male guppies)
my aquarium
Qualitative Data (Observations):
9-18-13
9-18-13
- added clam to tank
- fish tend to swim near top
- added 2 more plants
- ammonia spike(2.0)
- water change
- fish disappeared at the beginning of class then reappeared at the end of class
- water=clear
- plants look healthy
- nitrite shot up to 5.0 mg/l
- water=clear
- plants look healty
- ammonia=.50mg/l
- ammonia is 0
- nitrite shot up to 5.0mg/l
- fish in filtration (still alive) :)
- fish look good
- water=clear
- fish ate today
- ammonia=good
- nitrite=bad
- ammonia=good
- nitrite=5.0mg/l
- plants look healthy and green
- fish still swim near the top
Conclusion Questions:
1. Ammonia:
it comes from fish waste and excess food; extremely toxic to
fish; important to check because if not monitored high levels can lead
to fish death.

Nitrite: comes from Nitrosomonas bacteria in an aquarium; not as toxic as ammonia but still toxic; important to check because if not monitored high levels can lead to fish death.
Nitrate: comes from Nitrobactor bacteria in an aquarium; not very toxic in an aquarium and good for plants; important to check because if not monitored high levels can lead to fish death.
Temperature: is important because some fish can only live in certain temperatures
pH: The acidity or baseness of a liquid where 7 is neutral. From 6.8 to 7.8 is the ideal pH for fish. 4= fish reproduction is affected and 3= fish death
Conductivity: a measure of how well a liquid conducts electricity, its important because it measures nutrients in the water(?).
Dissolved Oxygen: Measures how much oxygen is in the water; its important to check because if you're fish don't get enough oxygen they can die.
Hardness: a measure of dissolved minerals in water
Alkalinity: the buffering capacity of water; it's important because it tells how well a liquid can resist changes
Chlorine: Chlorine is a disinfectant in tap water that is very harmful to fish gills.
2. The nitrogen cycle starts when
you feed the fish. The fish's waste turns to ammonia which can and will
kill it if the level gets to high. It's as poisonous to your fish as
carbon monoxide is to humans. Luckily, after a few days ammonia eating
bacteria starts to form eating away at the ammonia. This bacteria is
called nitrosomonous bacteria and produces Nitrite as a result of "eating" the ammonia. Then another bacteria starts to form called Nitrobacter.
It gets rid of the Nitrite which is less poisonous than ammonia, but
still bad for your fish. Nitrate is formed as a result of the
Nirtobacter. Nitrate can be absorbed by the live plants in your aquarium
as a fertilizer. Over time, the live plant can improve water quality.

3. My water quality has changed many times over the course of the aquarium
project. Specifically the nitrite and ammonia levels. I have a small
aquarium so it requires more maintenance than I have time for. So what I'm
having problems keeping under control are the ammonia and nitrite. Some
days it will be fine and not have a problem, but on days like 10-1-13, I
have a large problem with these levels.
4. The oxygen/ carbon dioxide cycle is important because it is what helps regulate the amount of oxygen and carbon dioxide in an aquatic ecosystem. During the day, plants and some plankton photosynthesize to make oxygen in the water. Fish and other organisms breathe in the O2 produced by the plants and breathe out the CO2. The CO2 is then absorbed by the plants and plankton and the cycle starts again. However at night, plants can't photosynthesize without light so CO2 levels rise until morning when the sun starts to provide light.
5. The problems I had with this project were keeping the ammonia and nitrites down and getting them both to 0 mg/L. To solve these problems you just have to be patient. Let the cycle happen while keeping up with routine water changes and the aquarium will stabilize itself.
6. What I learned was, how to take care of fish, keeping a journal of observations and data, making, watching and caring for an aquarium, there are different cycles that affect the stability of the aquarium, and how to test for water quality.
7. The most interesting thing that happened was watching my fishes' behavior. There were 4 male guppies, and they just kept around the top of the aquarium between the plants. The worst thing was having spikes in ammonia and nitrite. It really scared me because the levels got really high sometimes and I wouldn't have enough time to do a water change, I thought I would loose all of them. However, they proved to be real hardy fish and made it through. The weirdest thing that happened was when I'd be looking through my tank and I couldn't find one guppy for about 3 days. I looked in the filter and I found him in a pocket of flowing, filtered water (he was still alive).
8. My favorite part of the project was just having the aquarium itself. It gave me something to look forward to everyday.
9. Advice I'd give to next year's students would be to not use a small aquarium. I'd be best for you and your fish to have a larger aquarium. To make this project better, I'd say have more time with the aquariums.
Water Quality Analysis Chart
| Date | Ammonia | Nitrite | Temperature | |||
| 9/4/2013 | 1.0 mg/L | 0 mg/L | 22⁰C/ 77⁰F | |||
| 9/5/2013 | .25 mg/L | .50 mg/L | 25⁰C/ 76⁰F | |||
| 9/9/2013 | .50 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/10/2013 | .25 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/11/2013 | 1.0 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/12/2013 | .50 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/17/2013 | 1.0 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/18/2013 | .25 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/19/2013 | 2.0 mg/L | 0 mg/L | 27⁰C/ 81⁰F | |||
| 9/23/2013 | .50 mg/L | 0 mg/L | 27⁰C/ 81⁰F | |||
| 9/24/2013 | .50 mg/L | 0 mg/L | 26⁰C/ 80⁰F | |||
| 9/30/2013 | 1.0 mg/L | 1.0 mg/L | 27⁰C/ 81⁰F | |||
| 10/1/2013 | .50 mg/L | 5.0 mg/L | 30⁰C/ 82⁰F | |||
| 10/7/2013 | 0 mg/L | 5.0 mg/L | 26⁰C/ 80⁰F | |||
| 10/9/2013 | 0 mg/L | 1.0 mg/L | 27⁰C/ 81⁰F | |||
| 10/10/2013 | .25 mg/L | 5.0 mg/L | 27⁰C/ 81⁰F | |||
| 10/15/2013 | 0 mg/L | 5.0 mg/L | 28⁰C/ 81⁰F | |||
| 10/16/2013 | 0 mg/L | 0 mg/L | 27⁰C/ 81⁰F |
 |
| Chart A: Aqua Check |
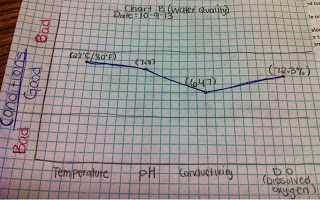 |
| Chart B: Water Quality |
 |
| Chart C: Nitrogen Cycle |

No comments:
Post a Comment